Quality control at MyoGen starts long before the actual production of our products ever begins, with regular maintenance and control of all the machinery and hardware involved in the manufacturing process.
The development and production processes are closely monitored. During product manufacturing, each step of the process is checked for compliance with pharmaceutical grade standards.
Our laboratory in India utilizes the latest chromatographs. Every time raw material is procured, it undergoes comparison with the standard, post which it goes into manufacturing after satisfying all criteria. GMP certification also assures consumers of enhanced standards of production.
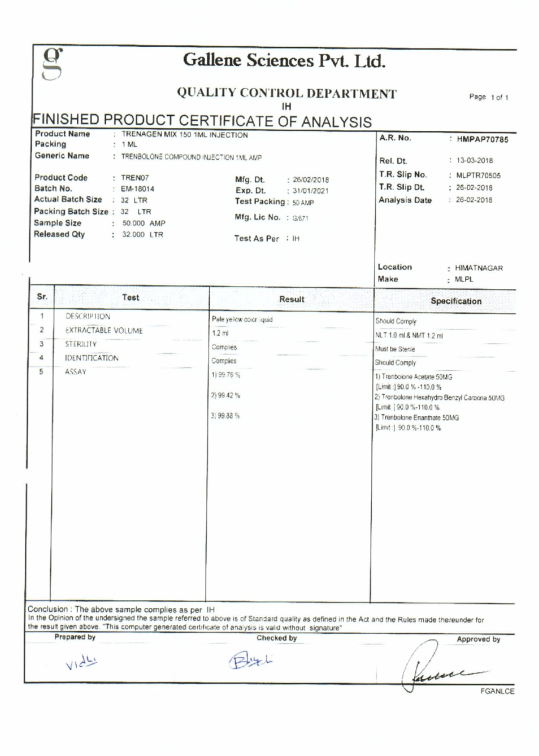
Once the products are manufactured, each batch is submitted to rigorous laboratory testing to ensure that each product is in exact accordance with its specifications. Our products are also subjected to random quality testing to guarantee product quality. The test results are shared so that consumers have a clear understanding of the product they intend to purchase.
The first quality reports available on April 2018 are injectables: DecaGen 250 (see the test), SustaGen 250 (see the test), StanoGen 50 (see the test), TrenaGen Mix 150 (see the test) and TestoGen 250 (see the test).
Update May 2018, orals quality reports are available: AnaGen (see the test), DianaGen (see the test), NolvaGen (see the test), OxaGen (see the test), StanoGen 10 (see the test) and TuriGen (see the test).
TECHNOLOGY & INNOVATION
MyoGen’s success lies in understanding market dynamics and buyer behavior and responding promptly. The company is adept at tapping the pulse of the market. We analyze buyer sentiments and behavior and the findings have helped us make continuous and remarkable investments in R&D.
MyoGen has been successful in keeping up with latest technology trends and innovation, adhering to quality standards and offering products well-differentiated from competitors in terms of quality and cost.
Backed by research, expertise and experience spanning over years, MyoGen has kept up with innovations pertaining to procurement and quality of raw materials, laboratory equipment, drug synthesis, product packaging, and anticounterfeiting techniques to keep competition at bay.
https://youtu.be/W8_Qn4m-Qr4
ANTI COUNTERFEITING
MyoGen uses sophisticated technology to distinguish its products from fake counterparts. The company makes it easier for consumers to differentiate its anabolic steroids from fake products.
Unique Serial Number: All ampoules or tablet packs have a unique serial number that can be checked on the company’s website, ensuring originality and full traceability of the product. Check my products
Ampoules: Our injectable products come in glass ampoules to ensure your product is kept in a perfect sanitary condition. The ampoules are colour coded specifically to match product, dosage and ester type.
Tablets: When it comes to tablets, MyoGen’s products come in blister packs. Counterfeiters sell tablets in bottles as the production process is cheaper, and so is shipping.
Seals: Oral tablets are sealed very carefully to keep out any bacteria or air. Most of the counterfeiters use manual equipment and the top of the seals is usually very loose making it easy to remove. MyoGen’s seals have proper edges and are uniformly attached. The brand name is printed on i
Leaflet: Each MyoGen product is packaged together with its own product information sheet. This leaflet contain an additional counterfeit deterrence system and an anti-counterfeit symbol.
GOOD MANUFACTURING PROCESS
The Quality Management System ensures that every product manufactured and distributed by MyoGen abides by good practices and adheres to international quality standards.
There are clearly defined standards and procedures and that makes MyoGen GMP approved. ”GMP” stands for Good Manufacturing Process.
This is an international quality standard system, which ensures that products are produced and controlled in accordance with quality standards, specifications and requirements appropriate for their intended uses.


Steroidify Rep
“taz10” for 10% off
PM for link to order
The development and production processes are closely monitored. During product manufacturing, each step of the process is checked for compliance with pharmaceutical grade standards.
Our laboratory in India utilizes the latest chromatographs. Every time raw material is procured, it undergoes comparison with the standard, post which it goes into manufacturing after satisfying all criteria. GMP certification also assures consumers of enhanced standards of production.
Once the products are manufactured, each batch is submitted to rigorous laboratory testing to ensure that each product is in exact accordance with its specifications. Our products are also subjected to random quality testing to guarantee product quality. The test results are shared so that consumers have a clear understanding of the product they intend to purchase.
The first quality reports available on April 2018 are injectables: DecaGen 250 (see the test), SustaGen 250 (see the test), StanoGen 50 (see the test), TrenaGen Mix 150 (see the test) and TestoGen 250 (see the test).
Update May 2018, orals quality reports are available: AnaGen (see the test), DianaGen (see the test), NolvaGen (see the test), OxaGen (see the test), StanoGen 10 (see the test) and TuriGen (see the test).
TECHNOLOGY & INNOVATION
MyoGen’s success lies in understanding market dynamics and buyer behavior and responding promptly. The company is adept at tapping the pulse of the market. We analyze buyer sentiments and behavior and the findings have helped us make continuous and remarkable investments in R&D.
MyoGen has been successful in keeping up with latest technology trends and innovation, adhering to quality standards and offering products well-differentiated from competitors in terms of quality and cost.
Backed by research, expertise and experience spanning over years, MyoGen has kept up with innovations pertaining to procurement and quality of raw materials, laboratory equipment, drug synthesis, product packaging, and anticounterfeiting techniques to keep competition at bay.
https://youtu.be/W8_Qn4m-Qr4
ANTI COUNTERFEITING
MyoGen uses sophisticated technology to distinguish its products from fake counterparts. The company makes it easier for consumers to differentiate its anabolic steroids from fake products.
Unique Serial Number: All ampoules or tablet packs have a unique serial number that can be checked on the company’s website, ensuring originality and full traceability of the product. Check my products
Ampoules: Our injectable products come in glass ampoules to ensure your product is kept in a perfect sanitary condition. The ampoules are colour coded specifically to match product, dosage and ester type.
Tablets: When it comes to tablets, MyoGen’s products come in blister packs. Counterfeiters sell tablets in bottles as the production process is cheaper, and so is shipping.
Seals: Oral tablets are sealed very carefully to keep out any bacteria or air. Most of the counterfeiters use manual equipment and the top of the seals is usually very loose making it easy to remove. MyoGen’s seals have proper edges and are uniformly attached. The brand name is printed on i
Leaflet: Each MyoGen product is packaged together with its own product information sheet. This leaflet contain an additional counterfeit deterrence system and an anti-counterfeit symbol.
GOOD MANUFACTURING PROCESS
The Quality Management System ensures that every product manufactured and distributed by MyoGen abides by good practices and adheres to international quality standards.
There are clearly defined standards and procedures and that makes MyoGen GMP approved. ”GMP” stands for Good Manufacturing Process.
This is an international quality standard system, which ensures that products are produced and controlled in accordance with quality standards, specifications and requirements appropriate for their intended uses.


Steroidify Rep
“taz10” for 10% off
PM for link to order
Last edited: